Advances in Dental Materials
The degradation of dental fillings poses a significant challenge, incurring high costs for repeated treatments and impacting millions globally; this underscores the urgent need for more durable materials. The authors use nano-FTIR to analyze dental materials at the molecular level providing detailed insights into the chemical composition of dental composites and adhesives. It enables precise maps of the interfaces between different components in fillings, crucial for assessing bonding effectiveness and mechanical properties. The study also applies FTIR imaging spectroscopy to various adhesives, capturing high-resolution reflectance images at different wavenumbers and detailing molecular resonances. Through detailed spectral analysis, researchers can identify specific chemical vibrations that reveal material composition and integrity, which are essential for enhancing the clinical performance and durability of dental restorations.nano-FTIR insights will guide the development of dental materials tailored to resist shrinkage and microleakage, enhancing restoration longevity.
This measurement was realized with the IR-neaSCOPE+s.
Further reading:
Beddoe et al., Acta Biomaterialia 168, 309-322, (2023)
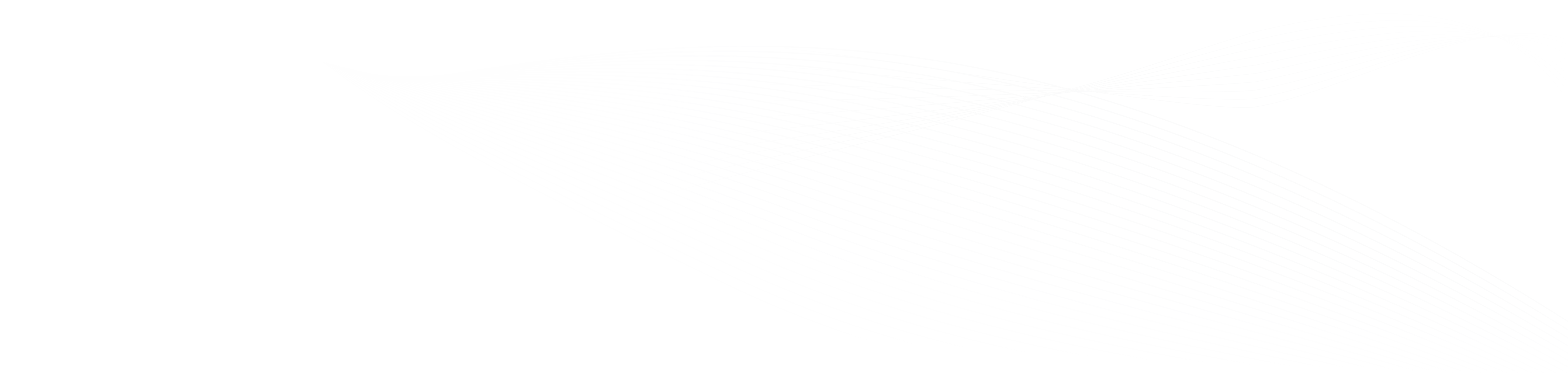
Single Protein IR Nanospectroscopy
The article from the Institute for Molecular Science discusses a breakthrough in observing single proteins using nano-FTIR, enabling the detection of infrared vibrational spectra of a single protein. This advancement allows for analyzing extremely small samples, which was previously challenging with conventional methods. The research is significant for understanding protein functions and interactions, and it opens new possibilities in nanoscale infrared spectroscopy applications across various fields. The study represents a major step in ultra-sensitive and high-resolution imaging using mid-infrared light.nano-FTIR, as demonstrated in the study, opens up diverse applications in fields like molecular biology for precise protein analysis, materials science for nanomaterial characterization, and medical diagnostics for identifying molecular markers in diseases.
This measurement was realized with the IR-neaSCOPE+s.
Further reading:
Nishida et al., Nano Lett. 2024
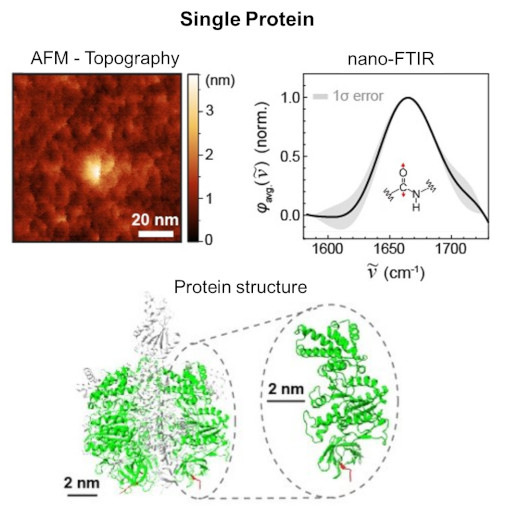
nano-Vesicle Tumor Diagnostic
Cells communicate by releasing tiny, membrane-bound vesicles, pivotal in transmitting bioactive molecules. These vesicles are not just cellular messengers; they play a crucial role in the development and spread of tumors. One intriguing aspect of these vesicles is their protein composition, which changes in correlation with the severity of malignancy. This study dives into this world of microscopic communication using nano-FTIR to examine the subtle differences in individual small extracellular vesicles related to cancer.Xue et al. discovered that as a tumor becomes more malignant, the ratio of amide I/II absorption in these vesicles increases. Ratio which corelates directly with the decrease in a-helix and random coil structures and increase of ß-sheet and ß-turn structures. The use of nano-FTIR spectroscopy is proving to be invaluable in detecting these small but critical variations. This research paves the way for a novel, noninvasive approach to understanding cancer's progression. It holds the promise of uncovering new biomarkers for cancer, potentially revolutionizing early detection methods.
This measurement was realized with the IR-neaSCOPE+s.
Further reading:
Xue et al., J. Am. Chem. 144, 20278 (2022)
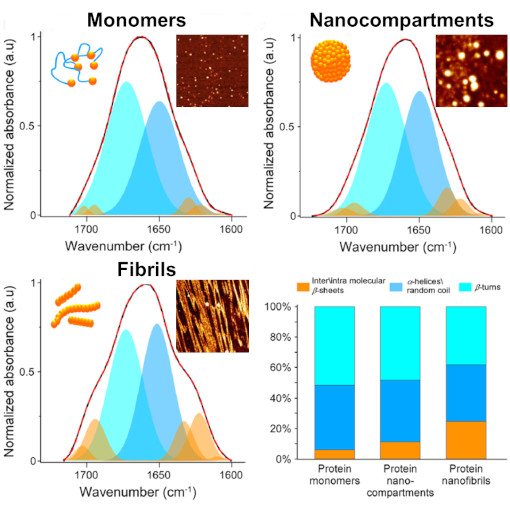
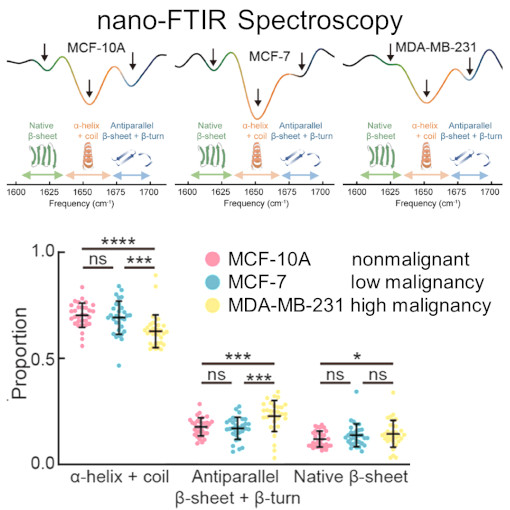
Nano-to-Micro Silk Fiber Morphing
Silk is a uniquely strong biomaterial, remarkable for its simple protein components. Despite numerous attempts, replicating silk synthetically remains a challenge. A groundbreaking discovery in silk fiber production was the formation of nanoscale compartments. These compartments facilitate a reversible transformation of silk proteins. They transition from their native structure into crystalline ß-sheets, a process that can be reversed. This reversibility is key to modifying the final mechanical properties of silk, opening doors to custom-tailored silk products. This customization is achieved by controlling the silk proteins' structural changes and their self-assembly into larger molecular formations.nano-FTIR spectroscopy has enabled researchers to closely examine protein structures at the nanometer level. This technique is especially beneficial for analyzing protein complexes and the stages of protein aggregation into fibers. It combines the morphological insights from AFM with the detailed structural information from FTIR spectroscopy. The team conducted qualitative tests with nano-FTIR spectra on different protein structures - from individual monomers to nanocompartments and fibrils - confirming the effectiveness of their method.This synergy of nano-FTIR spectroscopy and atomic force microscopy has been pivotal in deciphering the reversible transitions of silk proteins. It's a cornerstone in the journey towards developing more sophisticated and versatile silk-based materials.
This measurement was realized with the IR-neaSCOPE+s.
Further reading:
Eliaz et al., Nature Communications 13, 7856 (2022)
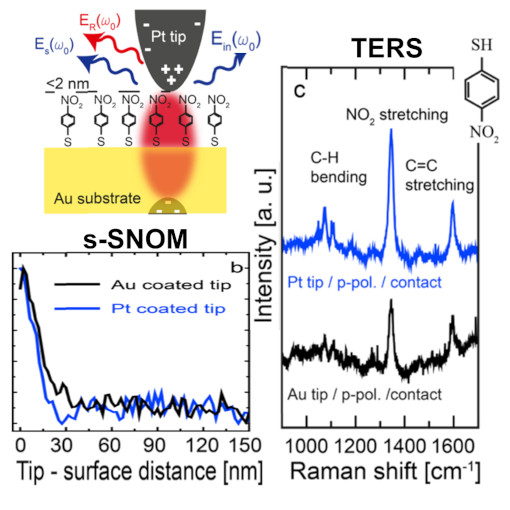
Combined TERS and s-SNOM
Tip-enhanced Raman spectroscopy (TERS) and scattering-type scanning near-field optical microscopy (s-SNOM) enable optical imaging with a spatial resolution far below the diffraction limit of light. Although s-SNOM records the elastically scattered light (yielding information about the local refractive index and absorption), in TERS, the Raman scattered light is detected, which provides, for example, chemical information. Here, we introduce a combined TERS and s-SNOM setup for correlative studies of tip-enhanced elastically scattered and Raman scattered light. Comparing s-SNOM and TERS signals, we demonstrate a qualitative correlation between the tip-enhanced elastic and tip-enhanced Raman scattered light. Thus, recording the tip-enhanced elastically scattered light enables a fast and reliable TERS alignment. Further, we demonstrate experimentally and by simulations that Pt-coated silicon tips can be used for TERS in gap-mode configuration.
This unique technological marriage could be employed for correlative analyses of structural, chemical, and photonic sample properties.
This measurement was realized with the IR-neaSCOPE+TERs.
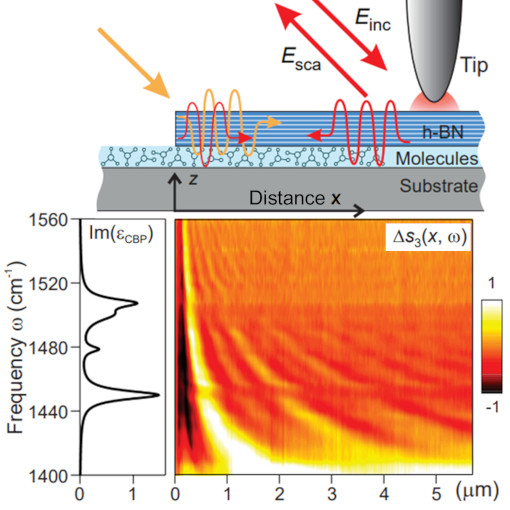
Phonon Polaritons & Organic Molecules
Light plays an essential role in modern science and technology, with applications ranging from fast optical communication to medical diagnosis and laser surgery. In many of these applications, the interaction of light with matter is of fundamental importance. The images obtained in this work reveal that the interaction between infrared light and molecular vibrations can be so strong that eventually the material properties are modified, such as conductivity and chemical reactivity. This effect, called vibrational strong coupling, could be used in the future for development of ultrasensitive spectroscopy devices or to study quantum aspects of strong vibrational coupling that have been not accessible so far.
Infrared s-SNOM imaging reveals vibrational strong coupling between propagating phonon polaritons and small organic molecules. A phenomenon with high potential to control fundamental physical and chemical material properties.
This measurement was realized with the IR-neaSCOPE+s.
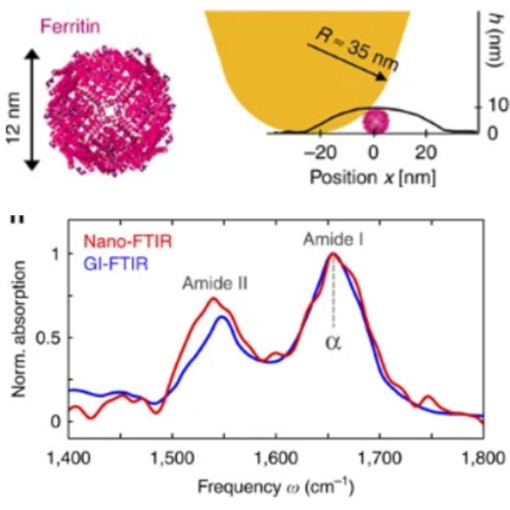
Secondary Structure of Single Proteins
The secondary structure of a proteins is highly relevant in the pathogenous mechanism leading to Alzheimer, Parkinson, and other neuro-degenerative diseases. Although a variety of methods have been developed to study the protein chemistry and structure, recognizing and mapping the secondary structure on the nanometer scale, or even with single protein sensitivity, is still a major challenge. nano-FTIR technology is used in this study to enabled nanoscale chemical imaging and probing of protein’s secondary structure with enormous sensitivity. In short, a sharp metalized tip is illuminated with a broadband infrared laser beam, and the backscattered light is analyzed with a specially designed Fourier transform spectrometer.
nano-FTIR probes the infrared spectroscopy and resolves the secondary structure of proteins complexes with diameter of 12 nm, 6 nm protein monolayer and even of 3 nm thin fibrils.
This measurement was realized with the IR-neaSCOPE+s.
Further reading:
Amenabar et al., Nature Communications 4, 2890 (2013)
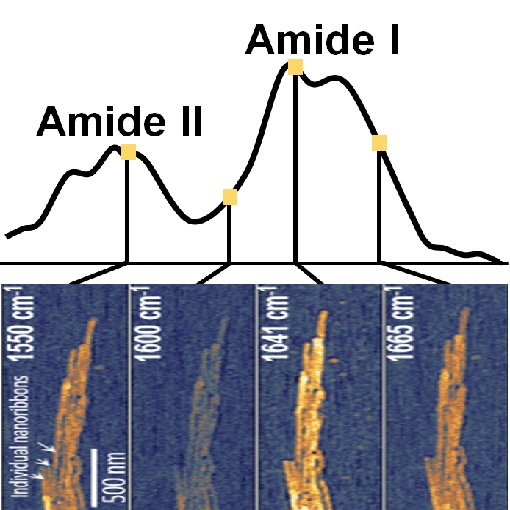
Mineralization of Protein Nanoribbons
Dental enamel is the hardest tissue created by vertebrates and exhibits complex nanoscale organizations of apatite crystals and protein nanoribbons. Previous studies using AFM and TEM show the formation of nanoribbons and even suggested their twisted structure. In this study, tapping AFM-IR+ provides additional chemically-specific information by measuring local infrared sample absorption. And indeed, absorption in the amide I and amide II bands verifies the protein composition of nanoribbons and provides insights into their α-helix/β-turn secondary structure. In addition to basic understanding of enamel growth, this study it is an important step towards synthesis of nanostructured materials using self-assembled organic framework.
Using low energy photons, tapping AFM-IR+ is highly suitable for nanoscale imaging and spectroscopy of fragile biological samples, such as protein, fibrils, lipids, viruses and DNA.
This measurement was realized with the IR-neaSCOPE.
Further reading:
Bai et al., PNAS 117, 19201-19208 (2020)